|
个人信息:Personal Information
副教授 硕士生导师
教师英文名称:Xiaoxi Li
教师拼音名称:lixiaoxi
所在单位:医学院
职称:副教授
毕业院校:分子细胞科学卓越创新中心
硕士生导师
学科:临床检验诊断学
-
(2) Li Xiaoxi, Ren Weijun, Liu Ling, Liu Chuan, Chen Xiang. A Critical Reassessment of Tumor Metastasis Simulation in Murine Models: Insights Into Methodological Advances and Biological Relevance. Advanced Therapeutics. 2025;8(12):e00277.
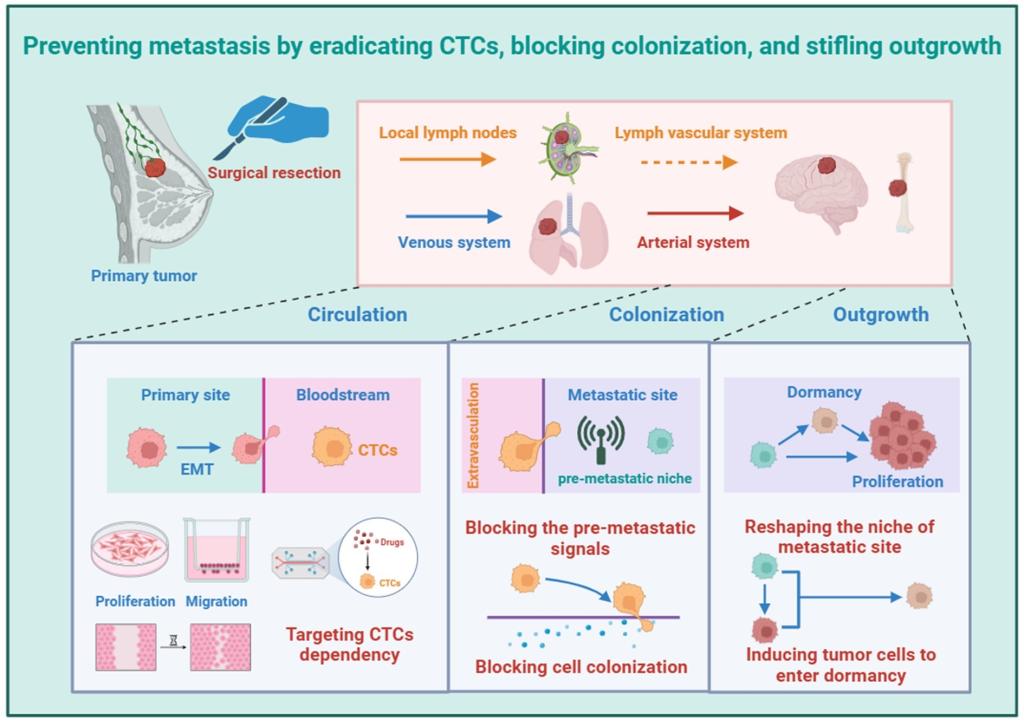
Abstract: Metastasis drives treatment failure and cancer mortality, yet preclinical studies still rely heavily on subcutaneous xenograft models that prioritize tumor growth over metastatic biology. This disconnection from clinical reality significantly contributes to the high failure rate of experimental therapies in trials. Here, current mechanistic insights is integrated into metastasis and critically assess transplantation models to inform rational model selection for metastasis research. Tumor transplantation models exhibit distinct dissemination patterns governed by implantation methodology rather than intrinsic tumor properties. Subcutaneous models, while technically accessible, predominantly assess primary tumor growth and fail to capture critical metastatic steps like intravasation, pre-metastatic niche formation, and organotropism. Orthotopic transplantation faithfully replicates native tissue microenvironments, enabling simultaneous assessment of tumor growth and metastatic potential. Intravascular models, while inducing rapid colonization, distort natural metastatic progression by skipping early dissemination stages. Metastatic site transplantation isolates microenvironmental impacts on tumor adaptation but fails to capture de novo metastatic initiation. Ultimately, three strategies is proposed for preventing metastasis: Eradicating circulating tumor cells, blocking colonization, and stifling outgrowth. This perspective catalyzes the strategic advancement of tumor metastasis models, thereby strengthening the reliability of preclinical findings and accelerating their clinical translation.
(1) Li Xiaoxi; Jiang Yong; Qian Hui ; Lymphoma dissemination is a pathological hallmark for malignant progression of B-cell lymphoma, Frontiers in Immunology, 2023, 14:1286411
Abstract: Extranodal lymphoma occurs in one-third of lymphoma patients and is a key indicator of the international prognostic index, associated with unfavorable outcomes. Due to the lack of ideal models, the causes and characteristics of extranodal lymphoma are greatly underexplored. Recently, we observed a high incidence of extranodal lymphoma in two types of mouse models with tropism for the brain and kidneys. These findings prompt us to rethink the pathological progression of lymphoma colonization in lymph nodes and non-lymphoid organs. Nodal lymphoma, primary extranodal lymphoma and secondary extranodal lymphoma should be biologically and clinically distinctive scenarios. Based on the observations in mouse models with extranodal lymphoma, we propose that lymphoma dissemination can be seen as lymphoma losing the ability to home to lymph nodes. The pathological process of nodal lymphoma should be referred to as lymphoma homing to distinguish it from benign hyperplasia. Lymphoma dissemination, defined as a pathological process that lymphoma can occur in almost any part of the body, is a key pathological hallmark for malignant progression of B-cell lymphoma. Reshaping cellular plasticity is a promising strategy to allow transformed cells to homing back to lymph nodes and re-sensitize tumor cells to treatment. From this perspective, we provide new insights into the pathological progression of lymphoma dissemination and its inspiration on therapeutic interventions. We believe that establishing extranodal lymphoma mouse models, identifying molecular mechanism governing lymphoma dissemination, and developing therapies to prevent lymphoma dissemination will become emerging topics for fighting relapsed and refractory lymphoma.

-
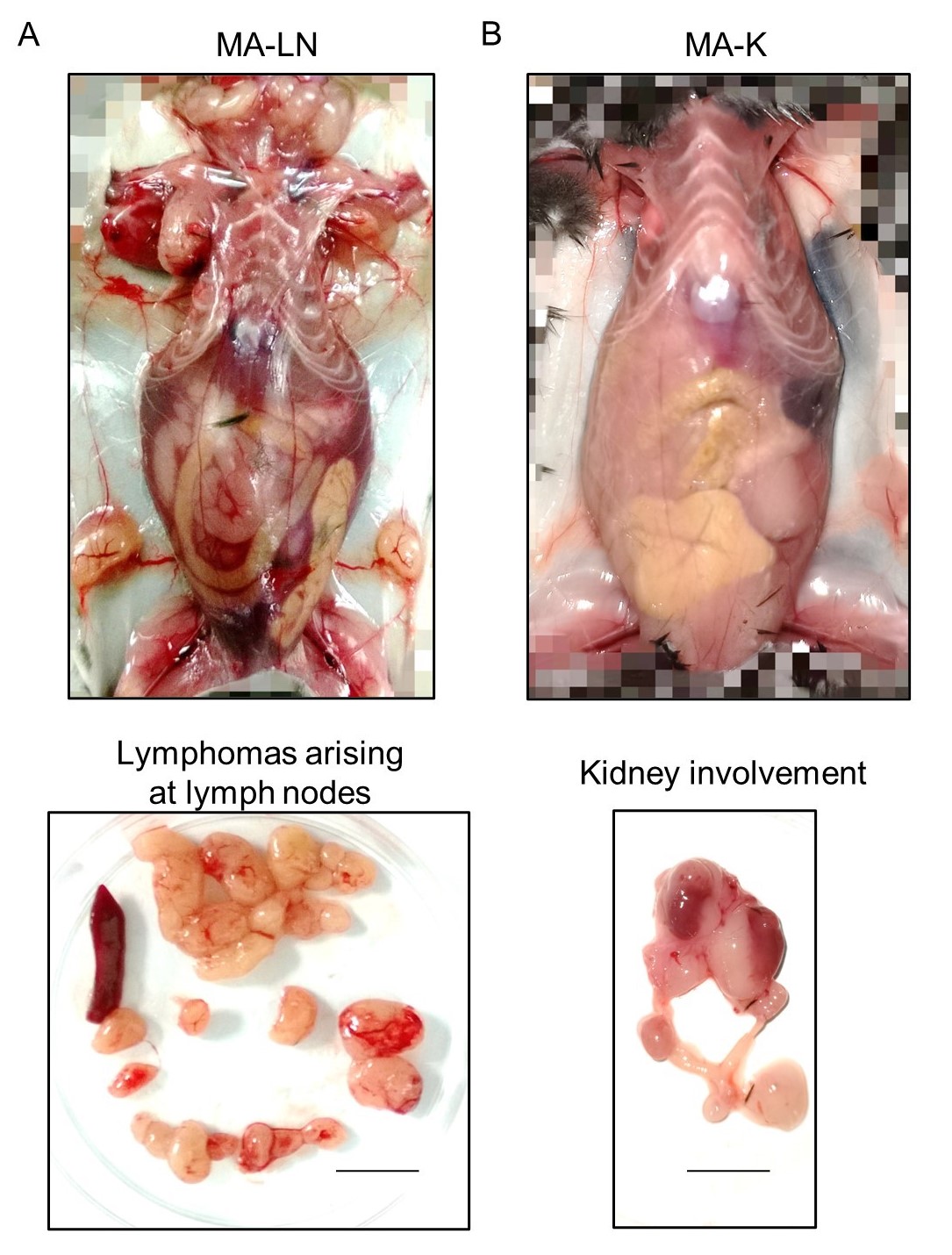
(1) Li Xiaoxi; Deng Minyao; Zhang Chenxiao; Luo Lingli; Qian Hui ; Establishment of a primary renal lymphoma model and its clinical relevance, Frontiers in Oncology, 2023, 13:1089187
Abstract: Extranodal dissemination is an important feature of aggressive B-cell lymphoma. Due to the lack of available animal models, the study on extranodal dissemination of lymphoma is greatly limited. Here, we identified a novel cell line, named MA-K, which originated from the Eμ-Myc;Cdkn2a-/- cell line, named MA-LN in this study. Compared to MA-LN, MA-K tended to disseminate in the kidney rather than lymph nodes in lymphoma transplantation model, resembling human primary renal lymphoma. The transcriptome analysis revealed that MA-K had undergone the transcriptional evolution during the culture. Specialized transcriptional pattern analysis, we proposed in this study, identified that FOXO1-BTG1-MYD88 pattern was formed in MA-K. Further analysis found that translation pathway was the most enriched pathway in specially expressed genes (SEGs) in MA-K. Among the SEGs, 3 up-regulated genes, RPLP2, RPS16 and MRPS16, and 5 down-regulated genes, SSPN, CD52, ANKRD37, CCDC82 and VPREB3, in MA-K were identified as promising biomarkers to predict the clinical outcomes of human DLBCL. Moreover, the joint expression of the 5-gene signature could effectively predict clinical outcomes of human DLBCL in triple groups. These findings suggested that the MA-K cell line had strong clinical relevance with human aggressive B-cell lymphoma. Moreover, the MA-K primary renal lymphoma model, as a novel syngenetic mouse model, will be greatly useful for both the basic research on lymphoma dissemination and preclinical efficacy evaluation of chemotherapy and immunotherapy.
-
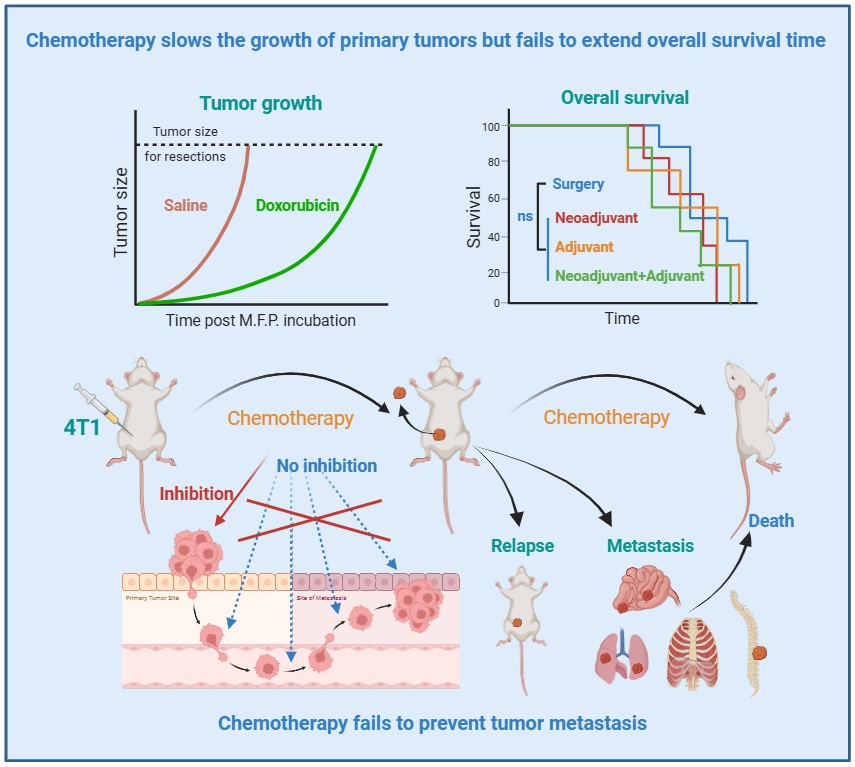
(2) Luo lingli, Liu Ling, Deng Mingyao, Jiang yong, Liu Chuan, Chen Xiang, Li Xiaoxi. The OtR tumor recurrence and metastasis model reveals doxorubicin‐induced tumor shrinkage doesn't guarantee prolonged survival. Animal Models and Experimental Medicine. 2026;00:1-8.
Abstract: Background: In preclinical research, tumor growth inhibition in subcutaneous models is frequently employed to evaluate therapeutic efficacy; however, such models often lack clinical translatability. Methods: To better approximate clinical reality, taking the case of doxorubicin treatment, we utilized an orthotopic transplant and resection (OtR) strategy to systematically assess the effects of neoadjuvant chemotherapy, adjuvant chemotherapy, and their combination on tumor growth, recurrence, and malignant progression. Results: Surprisingly, none of the treatments improved mouse survival, with adjuvant therapy even shortening it. Although neoadjuvant chemotherapy delayed preoperative tumor growth and all regimens reduced recurrence rates, none effectively prevented metastasis. Furthermore, all treatment groups exhibited weight loss, indicative of chemotherapy-induced cachexia. Conclusion: Collectively, these findings demonstrate that reduced tumor growth in preclinical mouse models does not necessarily translate into overall survival benefit. Our results emphasize the critical importance of prioritizing metastasis prevention over tumor growth inhibition as a key efficacy endpoint in antitumor drug evaluation.
(1) Li Xiaoxi; Luo Lingli; Qian Hui ; Improving the predictive accuracy of efficacy evaluation using tumor orthotopic transplant and resection model, Frontiers in Pharmacology, 2024, 15:1309876
Abstract: Preclinical efficacy evaluation and tumor drug sensitivity analysis are two main applications of efficacy evaluation. Preclinical efficacy evaluation is to predict whether candidate drugs or therapies may improve patient outcomes in clinical trials. Tumor drug sensitivity analysis is an approach for the personalized evaluation and optimization of approved anti-cancer drugs and treatment regimens. Overall survival (OS) is the gold standard to evaluate the outcome of drugs or therapies in both clinical trials and clinical treatment. Many efficacy evaluation models, such as cell model, tumor cell-line transplant model, patient-derived tumor xenograft model, tumor organoid model, have been developed to assess the inhibitory effect of tested drugs or therapies on tumor growth. In fact, many treatments may also lead to malignant progression of tumors, such as chemotherapy, which can lead to metastasis. Therefore, tumor growth inhibition does not necessarily predict OS benefit. Whether it can prevent or inhibit tumor recurrence and metastasis is the key to whether drugs and therapies can improve patient outcomes. In this perspective, we summarize the current understanding of the pathological progression of tumor recurrence and metastasis, point out the shortcomings of existing tumor transplant models for simulating the clinical scenario of malignant progression of tumors, and propose five improved indicators for comprehensive efficacy evaluation to predict OS benefit using tumor orthotopic transplant and resection model. Improvement in the accuracy of efficacy evaluation will accelerate the development process of anti-cancer drugs or therapies, optimize treatment regimens to improve OS benefit, and reduce drug development and cancer treatment costs.
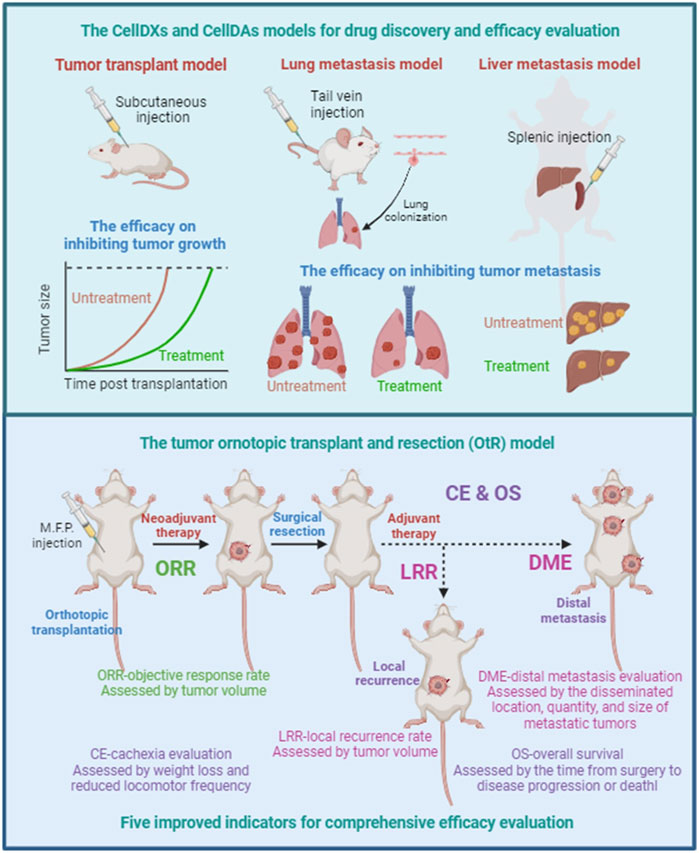
-
(1) Li Xiaoxi; Zhang Chenxiao*; Deng Minyao*; Jiang Yong; He Zhengjin; Qian Hui ; EFNB1 levels determine distinct drug response patterns guiding precision therapy for B-cell neoplasms, iScience, 2024, 27: 108667
Abstract: The multi-omics data has greatly improved the molecular diagnosis of B-cell neoplasms, but there is still a lack of predictive biomarkers to guide precision therapy. Here, we analyzed publicly available data and found that B-cell neoplasm cell lines with different levels of EFNB1 had distinctive drug response patterns of inhibitors targeting SRC/PI3K/AKT. Overexpression of EFNB1 promoted phosphorylation of key proteins in drug response, such as SRC and STMN1, conferring sensitivity to SRC inhibitor and cytotoxic drugs. EFNB1 phosphorylation signaling network was significantly associated with the prognosis of GCB-DLBCL patients. Moreover, EFNB1 levels were correlated with cell of origin (COO) scores, suggesting that EFNB1 is a quantitative indicator of cell differentiation. Ultimately, we proposed a model for the stratification of human B-cell malignancies and the implementation of targeted therapies based on EFNB1 levels. Our findings highlight that EFNB1 level is a promising biomarker for predicting drug response, COO and prognosis.
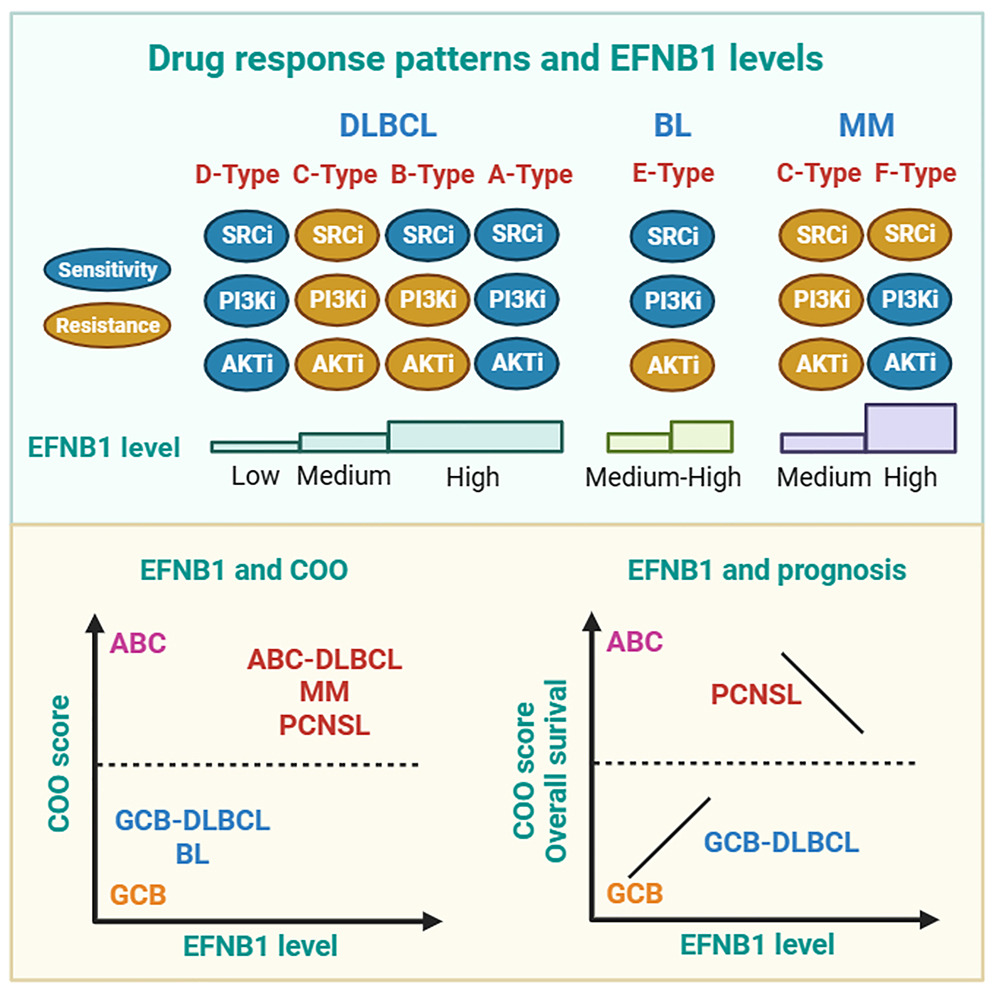
- 暂无内容
-
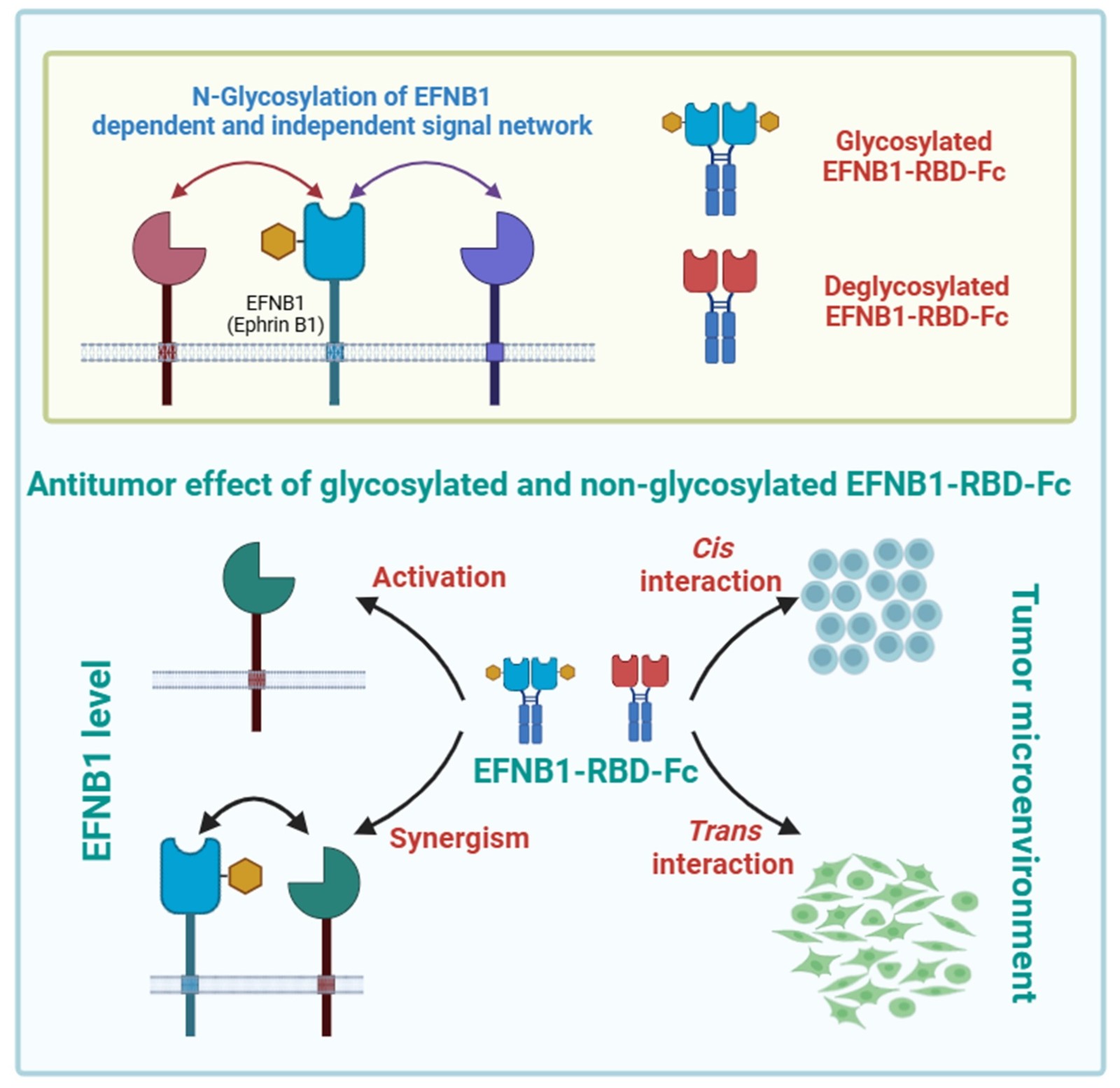
(1) Li Xiaoxi; Jiang Yong*; Deng Minyao*; Zhang Chenxiao; Tang Hua; N-glycosylation of ephrin B1 modulates its function and confers therapeutic potential in B-cell lymphoma, Journal of Biological Chemistry, 2025
Abstract: Given the pivotal role of the Eph-Ephrin signaling pathway in tumor progression, agonists or antagonists targeting Eph/Ephrin have emerged as promising anticancer strategies. However, the implications of glycosylation modifications within Eph/Ephrin and their targeted protein therapeutics remain elusive. Here, we identify that N-glycosylation within the receptor-binding domain (RBD) of ephrin B1 (EFNB1) is indispensable for its functional repertoire. Notably, compared to wild-type EFNB1, the glycosylation-deficient N139D mutant drastically diminishes the sensitivity of tumor cells to chemotherapeutic agents, suggesting the existence of both glycosylation-dependent and independent effects mediated by EFNB1. Transcriptomic analysis highlights immune response and oxidative phosphorylation as the primary signaling pathways modulated by glycosylation modifications. In coculture systems, the EFNB1-RBD-Fc recombinant protein, while inhibiting B-lymphoma cells, also exerts differential impacts on stromal cells depending on their glycosylation status. Furthermore, the efficacy of both glycosylated and non-glycosylated EFNB1-RBD-Fc is influenced by the endogenous EFNB1 levels within tumor cells. Taking together, this study demonstrates the complexity and multifaceted roles of glycosylation in modulating EFNB1 function. These findings underscore the need for a nuanced understanding of glycosylation patterns in Eph/Ephrin-targeted therapies to optimize their therapeutic potential.